GBSI Reports Encouraging Progress Toward Improved Research Reproducibility by Year 2020
 WASHINGTON, D.C., Feb. 19, 2017 - One year after the Global Biological Standards Institute (GBSI) issued its Reproducibility2020 challenge and action plan for the biomedical research community, the organization reports encouraging progress toward the goal to significantly improve the quality of preclinical biological research by year 2020. "Reproducibility2020 Report: Progress and Priorities," posted today on bioRxiv, identifies action and impact that has been achieved by the life science research community and outlines priorities going forward. The report is the first comprehensive review of the steps being taken to improve reproducibility since the issue became more widely known in 2012.
WASHINGTON, D.C., Feb. 19, 2017 - One year after the Global Biological Standards Institute (GBSI) issued its Reproducibility2020 challenge and action plan for the biomedical research community, the organization reports encouraging progress toward the goal to significantly improve the quality of preclinical biological research by year 2020. "Reproducibility2020 Report: Progress and Priorities," posted today on bioRxiv, identifies action and impact that has been achieved by the life science research community and outlines priorities going forward. The report is the first comprehensive review of the steps being taken to improve reproducibility since the issue became more widely known in 2012.
"By far the greatest progress over these few years has been in stakeholders recognizing the severity of the problem and the importance of taking active steps for improvement," said Leonard P. Freedman, PhD, president of GBSI. "Every stakeholder group is now addressing the issues, including journals, NIH, private funders, academicians and industry. That's crucial because there is not one simple fix--it is a community-wide problem and a community-wide effort to achieve solutions."
The report addresses progress in four major components of the research process: study design and data analysis, reagents and reference materials, laboratory protocols, and reporting and review. Moreover, it identifies the following broad strategies as integral to the continued improvement of reproducibility in biomedical research: 1) drive quality and ensure greater accountability through strengthened journal and funder policies; 2) create high quality online training and proficiency testing and make them widely accessible; 3) engage the research community in establishing community-accepted standards and guidelines in specific scientific areas; and 4) enhance open access to data and methodologies.
Research community stakeholders have responded with innovation and policy. The community is taking more steps to work together and to tackle the complexities of the reproducibility problem. The report highlights tangible examples of community-led actions from implementing new funding guidelines and accountability to tackling industry-wide research standards and incentives for compliance. The lessons learned from these early efforts will assist all stakeholders seeking to scale up or replicate successful initiatives.
"We are confident that continued transparent, global, multi-stakeholder engagement is the way forward to better, more impactful science," says Freedman. "We are calling on all stakeholders - individuals and organizations alike - to take action to improve reproducibility in the preclinical life sciences by joining an existing effort, replicating successful policies and practices, providing resources to replication efforts and taking on new opportunities."
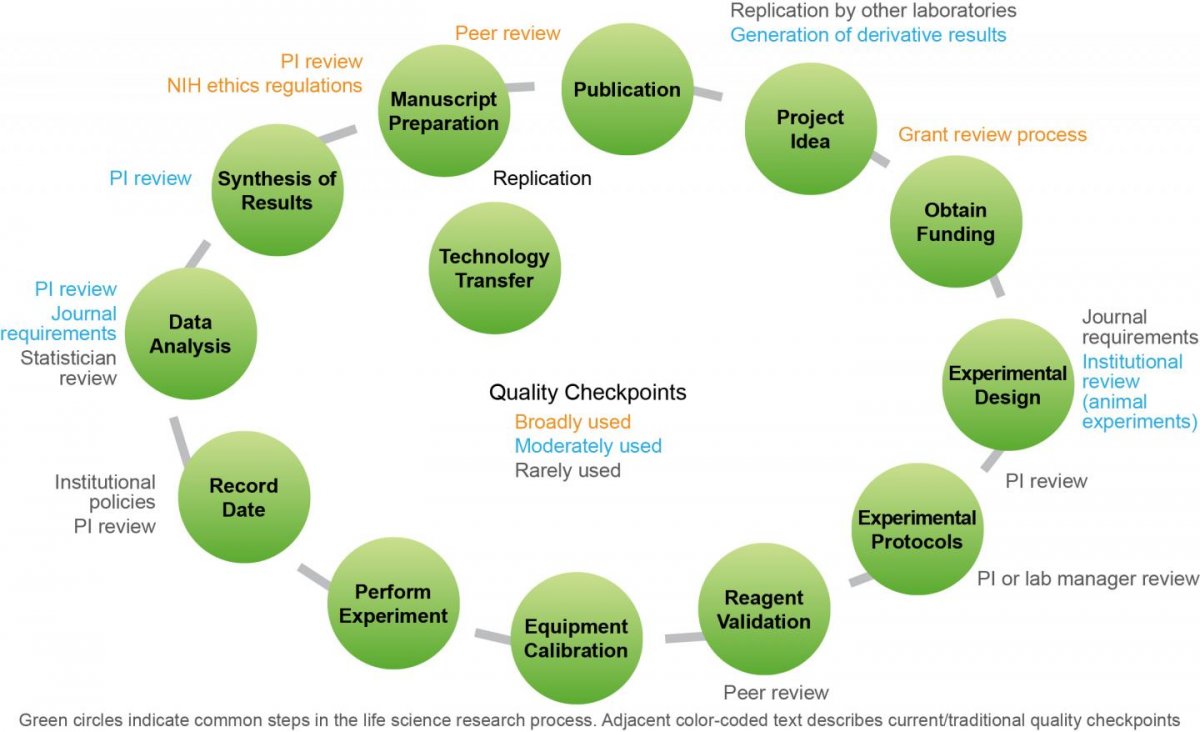
Actions Going Forward
The report contains specific actions that each stakeholder group can take to enhance reproducibility. In its leadership role, GBSI will:
- work with journals and funders to encourage policies that increase rigor, accountability and open access to data and methodologies,
- lead the effort toward improving the validation of reagents--particularly cells and antibodies-- and work with the research community to explore other scientific areas (e.g. stem cells and synthetic biology) where a greater emphasis on development of standards and best practices are needed to ensure quality and advance discovery,
- ensure high quality, accessible online training modules available to both emerging and experienced researchers who are eager to improve their proficiencies in new and evolving best practices; and
- periodically track and report on community-wide progress toward the 2020 goal.
Freedman introduced the new report at the AAAS 2017 Annual Meeting today during the session, "Rigor and Reproducibility One Year Later: How Has the Biomedical Community Responded?," hosted by GBSI. Freedman was joined by panelists Michael S. Lauer, M.D. of NIH; William G. Kaelin Jr., M.D. of the Dana-Farber Cancer Institute; and Judith Kimble University of Wisconsin-Madison.
"The research culture, particularly at academic institutions, must also seek greater balance between the pressures of career advancement and advancing rigorous research through standards and best practices," said Freedman, noting a major challenge still facing the community. "Additional leadership and community-wide support will be needed and we believe that the many initiatives described in this report add needed momentum to this emerging culture shift in science.
"The preclinical research community is full of talented, motivated people who care deeply about producing high-quality science. We are optimistic about the potential to improve reproducibility, and look forward to continuing to contribute to the effort."
###
About Global Biological Standards Institute (GBSI)
GBSI is an independent non-profit organization dedicated to enhancing the quality of biomedical research by advocating best practices and standards to accelerate the translation of research breakthroughs into life-saving therapies. GBSI was founded by ATCC. For more information, visit GBSI.org and Twitter @GBSIorg.
Media Contact
Carol Miller
[email protected]
202-667-2212
- Tags:
- Login to post comments