adverse drug reactions
See the following -
Chinese Melamine and American Vioxx: A Comparison
In contrasting China and America, pundits often cite our free and independent media as one of our greatest strengths, together with the tremendous importance which our society places upon individual American lives. For us, a single wrongful death can sometimes provoke weeks of massive media coverage and galvanize the nation into corrective action, while life remains cheap in China, a far poorer land of over a billion people, ruled by a ruthless Communist Party eager to bury its mistakes. But an examination of two of the greatest public-health scandals of the last few years casts serious doubt on this widespread belief.
- Login to post comments
CMS Goes Live with Blue Button - With Life and Cost Saving Applications for 53 Million Americans to Use
 On August 13 at the White House in Washington, D.C., the Office of American Innovation and the Center for Medicare and Medicaid Services (CMS) will host the first Blue Button 2.0 conference. This event will highlight CMS’ strong investment and leadership in Blue Button as a patient driven means for interoperability, cost-effective care and patient safety. Eight years after President Obama’s announcement of the Blue Button initiative to give Veterans, military beneficiaries and Medicare beneficiaries “easy access to their health information” with the use of a “Blue Button”, CMS Administrator Seema Verma took action with “Blue Button 2.0” so that 53 million Medicare beneficiaries can now make use of CMS approved patient facing Blue Button applications, turning a four-year history of claim data into actionable longitudinal health records to prevent costly medical errors, unnecessary redundant care or other harmful and wasteful care.
On August 13 at the White House in Washington, D.C., the Office of American Innovation and the Center for Medicare and Medicaid Services (CMS) will host the first Blue Button 2.0 conference. This event will highlight CMS’ strong investment and leadership in Blue Button as a patient driven means for interoperability, cost-effective care and patient safety. Eight years after President Obama’s announcement of the Blue Button initiative to give Veterans, military beneficiaries and Medicare beneficiaries “easy access to their health information” with the use of a “Blue Button”, CMS Administrator Seema Verma took action with “Blue Button 2.0” so that 53 million Medicare beneficiaries can now make use of CMS approved patient facing Blue Button applications, turning a four-year history of claim data into actionable longitudinal health records to prevent costly medical errors, unnecessary redundant care or other harmful and wasteful care.
- Login to post comments
FDA Issues RFQ for Large Scale EHR Study - Wants to Leverage VA's Open Source VistA EHR and Database for Research
 The Food and Drug Administration (FDA) yesterday issued a Request for Quotation (RFQ) for a large-scale electronic health record (EHR) system. This RFQ is very important as the objective is to develop a platform to support a critical project by the FDA's Division of Bioinformatics and Biostatistics (DBB) "to conduct research to assess the safety and surveillance of FDA regulated products through the FDA adverse event reporting systems..." Adverse drug reactions are one of the leading causes of death in the US, thus finding which drugs cause negative interactions is of vital importance. The project requires "use of the large electronic medical record (EMR) system..." The project is going to leverage the largest, most comprehensive, and clinically relevant medical records database, that of the US Department of Veterans Affairs (VA).
The Food and Drug Administration (FDA) yesterday issued a Request for Quotation (RFQ) for a large-scale electronic health record (EHR) system. This RFQ is very important as the objective is to develop a platform to support a critical project by the FDA's Division of Bioinformatics and Biostatistics (DBB) "to conduct research to assess the safety and surveillance of FDA regulated products through the FDA adverse event reporting systems..." Adverse drug reactions are one of the leading causes of death in the US, thus finding which drugs cause negative interactions is of vital importance. The project requires "use of the large electronic medical record (EMR) system..." The project is going to leverage the largest, most comprehensive, and clinically relevant medical records database, that of the US Department of Veterans Affairs (VA).
- The Future Is Open
- Login to post comments
FDA Seeks Data Mining Tool to Track Adverse Drug Reactions
The Food and Drug Administration is in the market for a data mining tool that will gather information on adverse reactions to vaccines and other drugs, according to solicitation documents posted Monday. Read More »
- Login to post comments
Humetrix to Showcase Medicare Approved Blue Button 2.0 Mobile Platform at HIMSS19
 At HIMSS 19 February 11-15 in Orlando, Florida, Humetrix will demo its iBlueButton 8.0 mobile health platform approved by the Centers for Medicare and Medicaid Services (CMS) to help millions of Americans covered by Medicare, Veterans, and military personnel in TRICARE receive safer and more cost-effective healthcare. A year after announcing the Medicare Blue Button 2.0 and MyHealthEData initiative at the HIMSS 18 conference, CMS Administrator, Seema Verma continues to emphasize the importance of "giving patients the necessary information they need to make the best decisions about their health care". Humetrix...has embraced this initiative from the start recognizing first and foremost that because Medicare beneficiaries are most at risk for medical errors and redundant tests across multiple providers they need to have access and use of their medical history wherever they receive care.
At HIMSS 19 February 11-15 in Orlando, Florida, Humetrix will demo its iBlueButton 8.0 mobile health platform approved by the Centers for Medicare and Medicaid Services (CMS) to help millions of Americans covered by Medicare, Veterans, and military personnel in TRICARE receive safer and more cost-effective healthcare. A year after announcing the Medicare Blue Button 2.0 and MyHealthEData initiative at the HIMSS 18 conference, CMS Administrator, Seema Verma continues to emphasize the importance of "giving patients the necessary information they need to make the best decisions about their health care". Humetrix...has embraced this initiative from the start recognizing first and foremost that because Medicare beneficiaries are most at risk for medical errors and redundant tests across multiple providers they need to have access and use of their medical history wherever they receive care.
- Login to post comments
Humetrix to Unveil its Medicare Approved iBlueButton 8.0 Mobile Platform at CES in Vegas
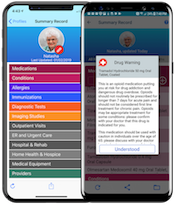 At CES, Humetrix will unveil at booth 43943 of the Tech West/Sands Expo Health & Wellness Marketplace, its iBlueButton 8.0 mobile health platform approved by the Centers for Medicare and Medicaid Services (CMS) for use by 53 million Americans covered by Medicare. A consumer life and cost saving digital health tool, iBlueButton 8.0 was introduced at the White House Blue Button Conference in August 2018 to illustrate how CMS approved applications powered by the Medicare Blue Button claim database can help millions of Americans in the Medicare program better manage their often complex and at times error-prone healthcare.
At CES, Humetrix will unveil at booth 43943 of the Tech West/Sands Expo Health & Wellness Marketplace, its iBlueButton 8.0 mobile health platform approved by the Centers for Medicare and Medicaid Services (CMS) for use by 53 million Americans covered by Medicare. A consumer life and cost saving digital health tool, iBlueButton 8.0 was introduced at the White House Blue Button Conference in August 2018 to illustrate how CMS approved applications powered by the Medicare Blue Button claim database can help millions of Americans in the Medicare program better manage their often complex and at times error-prone healthcare.
- Login to post comments
Opening Up the FDA
 The President's Executive Order on Open Government Data states, "Government information shall be managed as an asset throughout its life cycle to promote interoperability and openness, and, wherever possible and legally permissible, to ensure that data are released to the public in ways that make the data easy to find, accessible, and usable." Interestingly, the Department of Health and Human Services (HHS), which includes the Food & Drug Administration (FDA), has a tradition of expansive disclosure of information and/or data it generates or collects – contrary to current practices at the FDA. Hopefully, changes being made to 'open up' the FDA will start to accelerate. Read More »
The President's Executive Order on Open Government Data states, "Government information shall be managed as an asset throughout its life cycle to promote interoperability and openness, and, wherever possible and legally permissible, to ensure that data are released to the public in ways that make the data easy to find, accessible, and usable." Interestingly, the Department of Health and Human Services (HHS), which includes the Food & Drug Administration (FDA), has a tradition of expansive disclosure of information and/or data it generates or collects – contrary to current practices at the FDA. Hopefully, changes being made to 'open up' the FDA will start to accelerate. Read More »
Rethink Data, Transform Healthcare - Unlocking The Value Of Health Data
 We are all consumers of healthcare and therefore have a vested interest in its future. As an observation, being an outsider to this sector, the healthcare global system looks increasingly broken as the rate of change and complexity increases. At the same time, my empathy is with those people working inside the profession that provide high quality, compassionate healthcare, and support. But maybe more help is needed to handle the relentless challenges and changes at the edge. Read More »
We are all consumers of healthcare and therefore have a vested interest in its future. As an observation, being an outsider to this sector, the healthcare global system looks increasingly broken as the rate of change and complexity increases. At the same time, my empathy is with those people working inside the profession that provide high quality, compassionate healthcare, and support. But maybe more help is needed to handle the relentless challenges and changes at the edge. Read More »
- Login to post comments
Zoeticx Introduces TruRecord, Medical Prescribing Software to Address 2.2 Million Adverse Drug Reactions in the U.S
 Zoeticx, Inc., the developer of medical software that bridges the gap between medical data and quality patient care, today announced a crowd funding campaign for TruRecord, the first subscriber-based, medical prescribing software to leverage advanced DNA testing technology for optimal drug analysis and prevention of adverse drug reactions. The company is raising funds for a fall launch of TruRecord on medical crowd funding site Medstartr...TruRecord improves patient outcomes by reducing the 80,000 annual deaths due to drug adverse reactions, resulting in 289 billion spent annually on malpractice payouts.
Zoeticx, Inc., the developer of medical software that bridges the gap between medical data and quality patient care, today announced a crowd funding campaign for TruRecord, the first subscriber-based, medical prescribing software to leverage advanced DNA testing technology for optimal drug analysis and prevention of adverse drug reactions. The company is raising funds for a fall launch of TruRecord on medical crowd funding site Medstartr...TruRecord improves patient outcomes by reducing the 80,000 annual deaths due to drug adverse reactions, resulting in 289 billion spent annually on malpractice payouts.
- Login to post comments