Introducing OpenClinica Participate
 In clinical research, we all work towards better evidence-based, patient-centered health interventions. We all understand the importance of evidence. But how about patient-centered? We hear this phrase perhaps too often nowadays, but it’s more than just a buzzword. A patient-centered approach directs research toward questions that are important to patients so they can make more informed healthcare decisions. It measures the outcomes that are noticeable and important to patients, and produces results that help them weigh the value of healthcare.
In clinical research, we all work towards better evidence-based, patient-centered health interventions. We all understand the importance of evidence. But how about patient-centered? We hear this phrase perhaps too often nowadays, but it’s more than just a buzzword. A patient-centered approach directs research toward questions that are important to patients so they can make more informed healthcare decisions. It measures the outcomes that are noticeable and important to patients, and produces results that help them weigh the value of healthcare.
At OpenClinica, we think increasing the patient-centeredness of research is vital. Industry, NIH, FDA, and the general public seem to agree, and furthermore share our view that technology can increase patient engagement. This can happen by designing highly accessible, mobile technology to:
- Improve patient participation, motivation, and adherence
- Increase ability to meet recruitment goals, budget, and completion timelines
- Enable new designs that better target populations and/or more closely align with real-world use
I’d like to introduce our upcoming product, OpenClinica Participate, a tool tightly integrated with OpenClinica for engaging patients and collecting data directly from study participants.
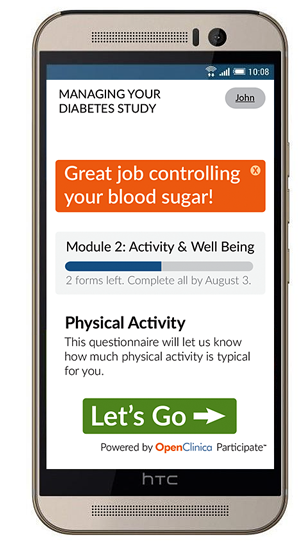 If you took OpenClinica's recent survey, you got a glimpse of what your forms for patient-reported outcomes could look like in OpenClinica Participate. Driven by a powerful forms engine based on proven open-source technology, the participant forms are simple, dynamic, mobile-focused, and platform-independent.
If you took OpenClinica's recent survey, you got a glimpse of what your forms for patient-reported outcomes could look like in OpenClinica Participate. Driven by a powerful forms engine based on proven open-source technology, the participant forms are simple, dynamic, mobile-focused, and platform-independent.
But it’s about a lot more than mobile-friendly forms. Patient expectations are rising while trial participation is shrinking. Clinical trials need to engage ‘Subjects’ as ‘Participants’ — by recruiting and retaining through real-time engagement and meeting the ‘anytime, anywhere’ expectations of a mobile, smartphone enabled world. It starts with an intuitive, action-oriented dashboard. Participants are greeted with a simple interface that motivates and focuses them on what they need to do today. The layout is responsive and knows whether you are using your phone, a tablet, or a traditional browser. Communication with the participant can occur through multiple channels — via the dashboard, through SMS, or via email, with options to help you make sure the right balance is struck between security and accessibility.
Last, and perhaps most important, Participate is tightly integrated with the OpenClinica EDC platform. The Participate solution allows you to design your study and forms using the OpenClinica study build tools you already know, while seamlessly capturing all your clinical and participant-reported outcomes in a single database. You can build skip patterns, repeats, and other logic into your participant forms just as you do with traditional OpenClinica CRFs, using the same rules engine. You can use scheduling rules to identify the next time participant feedback is required. As data is submitted by study participants, their activities become part of the same audit trail that tracks what your clinical users do. The data they submit can immediately be reviewed and extracted along with CRF data from other sources.
 Participate is extremely easy to adopt. As a modular add-on to your hosted OpenClinica Enterprise instance, it can be activated for any new study from within your study build screen. Forms are powered by the widely used open source enketo-express library and all editions of OpenClinica will support the widely-used OpenRosa API to let you run Enketo, ODK Collect, or any of a number of OpenRosa-compliant data capture clients. Participate utilizes a cloud-based Software-as-a-Service (SaaS) delivery model, so there is no costly and delay-inducing software deployment to worry about.
Participate is extremely easy to adopt. As a modular add-on to your hosted OpenClinica Enterprise instance, it can be activated for any new study from within your study build screen. Forms are powered by the widely used open source enketo-express library and all editions of OpenClinica will support the widely-used OpenRosa API to let you run Enketo, ODK Collect, or any of a number of OpenRosa-compliant data capture clients. Participate utilizes a cloud-based Software-as-a-Service (SaaS) delivery model, so there is no costly and delay-inducing software deployment to worry about.
| Introducing OpenClinica Participate was authored by Cal Collins and published in the OpenClinica blog. It is reprinted by Open Health News with permission. The original post can be found here. |
- Tags:
- Cal Collins
- clinical research
- clinical trials
- enketo-express library
- enterprise data capture
- evidence based medicine
- mobile focused
- ODK Collect
- Open Clinica 2015 (OC15)
- open source technology
- OpenClinica
- OpenClinica EDC platform
- OpenClinica Participate
- OpenRosa API
- patient adherence
- patient centered medical research
- patient centered medicine
- patient engagement
- patient motivation
- patient participation
- platform independent
- Software-as-a-Service (SaaS)
- Login to post comments