consumer reports
See the following -
Consumer Reports Dives into Healthcare Ratings
Consumer Reports magazine announced it's getting into the growing business of publishing public ratings of hospitals and private physicians. It's only the latest move in the trend toward improving consumer information online amid growing public and political pressure for transparency. Meanwhile, it will likely be part of another trend, as well--that of hospitals and physicians criticizing hospital and physician ranking sites. Read More »
- Login to post comments
Drugs You Don't Need For Disorders You Don't Have
One evening in the late summer of 2015, Lisa Schwartz was watching television at her Vermont home when an ad for a sleeping pill called Belsomra appeared on the screen. Schwartz, a longtime professor at Dartmouth Medical College, usually muted commercials, but she watched this one closely: a 90-second spot featuring a young woman and two slightly cute, slightly creepy fuzzy animals in the shape of the words “sleep” and “wake”...
- Login to post comments
Generic Drugs Don't Necessarily Mean Low Prices
NewsHour Weekend's Megan Thompson reports on the surprising disparity in pricing for generic drugs. Generics, generally thought to be cheap, can actually vary widely in price from pharmacy to pharmacy, causing some to skip medications altogether. Read More »
- Login to post comments
Humetrix to Unveil its Medicare Approved iBlueButton 8.0 Mobile Platform at CES in Vegas
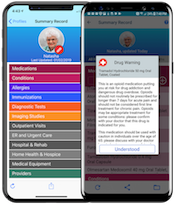 At CES, Humetrix will unveil at booth 43943 of the Tech West/Sands Expo Health & Wellness Marketplace, its iBlueButton 8.0 mobile health platform approved by the Centers for Medicare and Medicaid Services (CMS) for use by 53 million Americans covered by Medicare. A consumer life and cost saving digital health tool, iBlueButton 8.0 was introduced at the White House Blue Button Conference in August 2018 to illustrate how CMS approved applications powered by the Medicare Blue Button claim database can help millions of Americans in the Medicare program better manage their often complex and at times error-prone healthcare.
At CES, Humetrix will unveil at booth 43943 of the Tech West/Sands Expo Health & Wellness Marketplace, its iBlueButton 8.0 mobile health platform approved by the Centers for Medicare and Medicaid Services (CMS) for use by 53 million Americans covered by Medicare. A consumer life and cost saving digital health tool, iBlueButton 8.0 was introduced at the White House Blue Button Conference in August 2018 to illustrate how CMS approved applications powered by the Medicare Blue Button claim database can help millions of Americans in the Medicare program better manage their often complex and at times error-prone healthcare.
- Login to post comments
Medical Boards Behaving Badly
 Rats! I was all excited to write about virtual reality -- what with the long-anticipated release of the Oculus Rift -- or about how perhaps augmented reality is going to be the new reality, as some experts predict. Then Consumer Reports came out with a report that I had to write about: What You Don't Know About Your Doctor Could Hurt You. Long story short: chances are you don't know what you'd like to. Consumer Reports did a deep dive on the actions of the California medical board, obtaining their entire database of doctors on probation...
Rats! I was all excited to write about virtual reality -- what with the long-anticipated release of the Oculus Rift -- or about how perhaps augmented reality is going to be the new reality, as some experts predict. Then Consumer Reports came out with a report that I had to write about: What You Don't Know About Your Doctor Could Hurt You. Long story short: chances are you don't know what you'd like to. Consumer Reports did a deep dive on the actions of the California medical board, obtaining their entire database of doctors on probation...
- Login to post comments
ONC EHR Reporting Program RFI: A Public Health Perspective
 On August 24, 2018, the Office of the National Coordinator for Health Information Technology (ONC) released a Request for Information (RFI) related to the EHR Reporting Program. This RFI is required by the 21st Century Cures Act and its primary purpose is to gather ideas and suggestions related to how ONC might provide better information about Certified EHR Technology (CEHRT). Apparently, the initial intention was to create a "star rating" like the type used in Consumer Reports to use to rate EHRs, but that seems to have been abandoned in favor of some kind of measurement system. But it is far from clear exactly how this would be done.
On August 24, 2018, the Office of the National Coordinator for Health Information Technology (ONC) released a Request for Information (RFI) related to the EHR Reporting Program. This RFI is required by the 21st Century Cures Act and its primary purpose is to gather ideas and suggestions related to how ONC might provide better information about Certified EHR Technology (CEHRT). Apparently, the initial intention was to create a "star rating" like the type used in Consumer Reports to use to rate EHRs, but that seems to have been abandoned in favor of some kind of measurement system. But it is far from clear exactly how this would be done.
- Login to post comments
OpenNotes Introduces Advisory Board
 OpenNotes is pleased to announce that ten extraordinary advocates for health care quality and improvement are the founding members of the OpenNotes Advisory Board. OpenNotes is a national movement that urges doctors, nurses and other health care providers to share the notes they write with the patients they care for...
OpenNotes is pleased to announce that ten extraordinary advocates for health care quality and improvement are the founding members of the OpenNotes Advisory Board. OpenNotes is a national movement that urges doctors, nurses and other health care providers to share the notes they write with the patients they care for...
- Login to post comments
Serious Risks and Few New Benefits from FDA-Approved Drugs
Over the past year, the U.S. Senate and The New York Times have been investigating the failure of the nation’s auto safety regulators to protect citizens from cars with occasionally dangerous faulty devices. But neither august institution has paid attention to the Food and Drug Administration’s (FDA) failure to protect the 170 million Americans who take prescription drugs from adverse reactions that are killing more than 2,400 people every week. Annually, prescription drugs cause over 81 million adverse reactions and result in 2.7 million hospitalizations...
- Login to post comments