pacemakers
See the following -
Breaking The Chain
Patients with a new cardiac pacemaker have an advantage over patients who have received standard pacemakers: they can undergo MRI scans as a part of their care without the risk of adverse events. Read More »
- Login to post comments
FDA Partners with Sensato-ISAO and H-ISAC to Create Open Source Cybersecurity Intelligence Network and Resource
 The United States Food and Drug Administration (FDA) Center for Devices and Radiological Health (CDRH) has officially executed a tri-lateral agreement between the FDA, the Health Information Sharing and Analysis Center (H-ISAC) and the Sensato-Information Sharing and Analysis Organization (Sensato-ISAO), Sensato announced today. The goal of the agreement is "to ensure that essential medical device or healthcare cybersecurity vulnerability information can be shared with all stakeholders within the HPH Sector, including those who are not members of H-ISAC and Sensato-ISAO," according to a statement from the FDA. "This collaboration will help inform a common understanding of that risk threshold upon which exploit of a vulnerability might impact on patient safety and/or public health."
The United States Food and Drug Administration (FDA) Center for Devices and Radiological Health (CDRH) has officially executed a tri-lateral agreement between the FDA, the Health Information Sharing and Analysis Center (H-ISAC) and the Sensato-Information Sharing and Analysis Organization (Sensato-ISAO), Sensato announced today. The goal of the agreement is "to ensure that essential medical device or healthcare cybersecurity vulnerability information can be shared with all stakeholders within the HPH Sector, including those who are not members of H-ISAC and Sensato-ISAO," according to a statement from the FDA. "This collaboration will help inform a common understanding of that risk threshold upon which exploit of a vulnerability might impact on patient safety and/or public health."
- Login to post comments
Many Device Manufacturers May Miss Looming UDI Deadline
Vendors that sell medical devices to healthcare organizations will be scrambling to meet a mid-September deadline to get unique identifiers and meet requirements to match them with devices and accompanying software. Loftware, a vendor of unique device identifier labeling solutions, recently conducted a survey of about 120 medical device professionals and found that only 15 percent of respondents said they believe their organizations are ready for the September 24 deadline...
- Login to post comments
Soom Launches Mobile App That Notifies Patients, Caregivers and Nurses of Medical Device Recalls
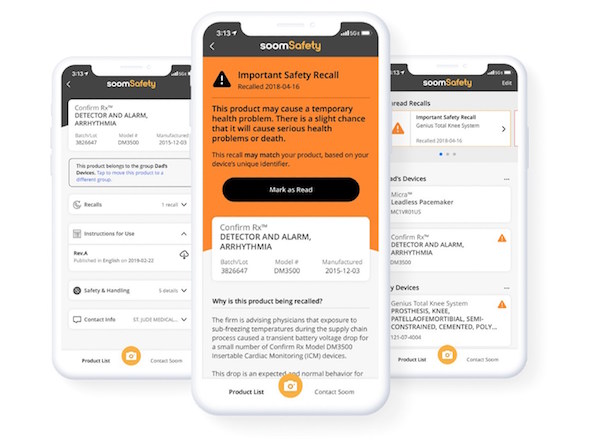 Soom, a pioneer in utilizing barcode and knowledge graph technologies to bridge information gaps between data sources and physical products, has introduced SoomSafety, an iOS mobile app that allows users to scan a medical device and receive instructions for use, safety and recall information directly from the device manufacturer and U.S. Food and Drug Administration (FDA). "We built SoomSafety to help patients and caregivers relying on implanted medical devices and using medical devices at home answer one critical question, 'Is this medical device safe to use?'" said Charlie Kim, President and CEO of Soom. "Our technology makes it possible to connect previously siloed medical device data, giving patients-and their caregivers-more proactive control over their health and safety."
Soom, a pioneer in utilizing barcode and knowledge graph technologies to bridge information gaps between data sources and physical products, has introduced SoomSafety, an iOS mobile app that allows users to scan a medical device and receive instructions for use, safety and recall information directly from the device manufacturer and U.S. Food and Drug Administration (FDA). "We built SoomSafety to help patients and caregivers relying on implanted medical devices and using medical devices at home answer one critical question, 'Is this medical device safe to use?'" said Charlie Kim, President and CEO of Soom. "Our technology makes it possible to connect previously siloed medical device data, giving patients-and their caregivers-more proactive control over their health and safety."
- Login to post comments
The Price of Wearable Craze: Personal Health Data Hacks
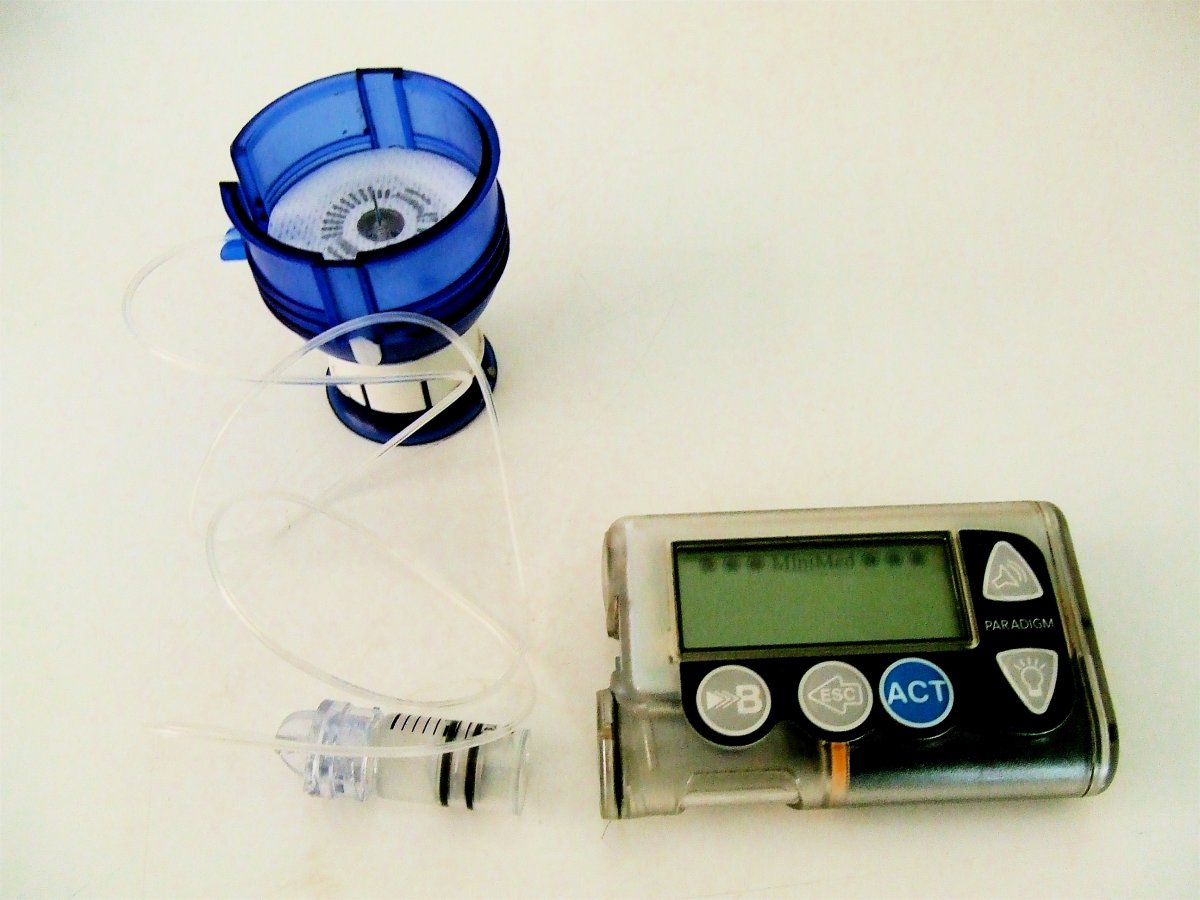 ...in a year when the world's largest technology, medical device and health-care firms are betting big and fast on wearable technology's role in delivering patients a more precise and cost-effective way to manage their health, experts are worried that the pace of updating data-privacy laws and building infrastructures with optimal levels of security doesn't match the speed of the market's technological rollout. The risks to consumers depend on what type of device they're wielding. In rare instances, weak links or endpoints in a cloud-based network powering something like a wearable insulin pump could be life threatening, as it opens the door to hackers tampering with them...
...in a year when the world's largest technology, medical device and health-care firms are betting big and fast on wearable technology's role in delivering patients a more precise and cost-effective way to manage their health, experts are worried that the pace of updating data-privacy laws and building infrastructures with optimal levels of security doesn't match the speed of the market's technological rollout. The risks to consumers depend on what type of device they're wielding. In rare instances, weak links or endpoints in a cloud-based network powering something like a wearable insulin pump could be life threatening, as it opens the door to hackers tampering with them...
- Login to post comments